China Customized 4-Hydroxyphenylboronic Acid Pinacol Ester 269409-70-3 Suppliers, Manufacturers, Factory - ALLYCHEM

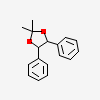
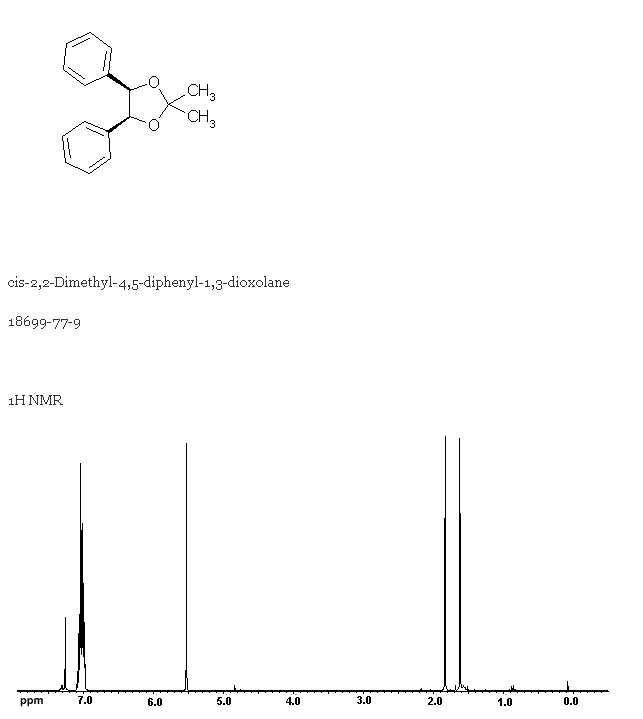
18699-77-9 1,3-Dioxolane, 2,2-dimethyl-4,5-diphenyl-, cis- C17H18O2 NMR,Molecular Structure, Molecular Formula, -Wörterbuch - guidechem.com
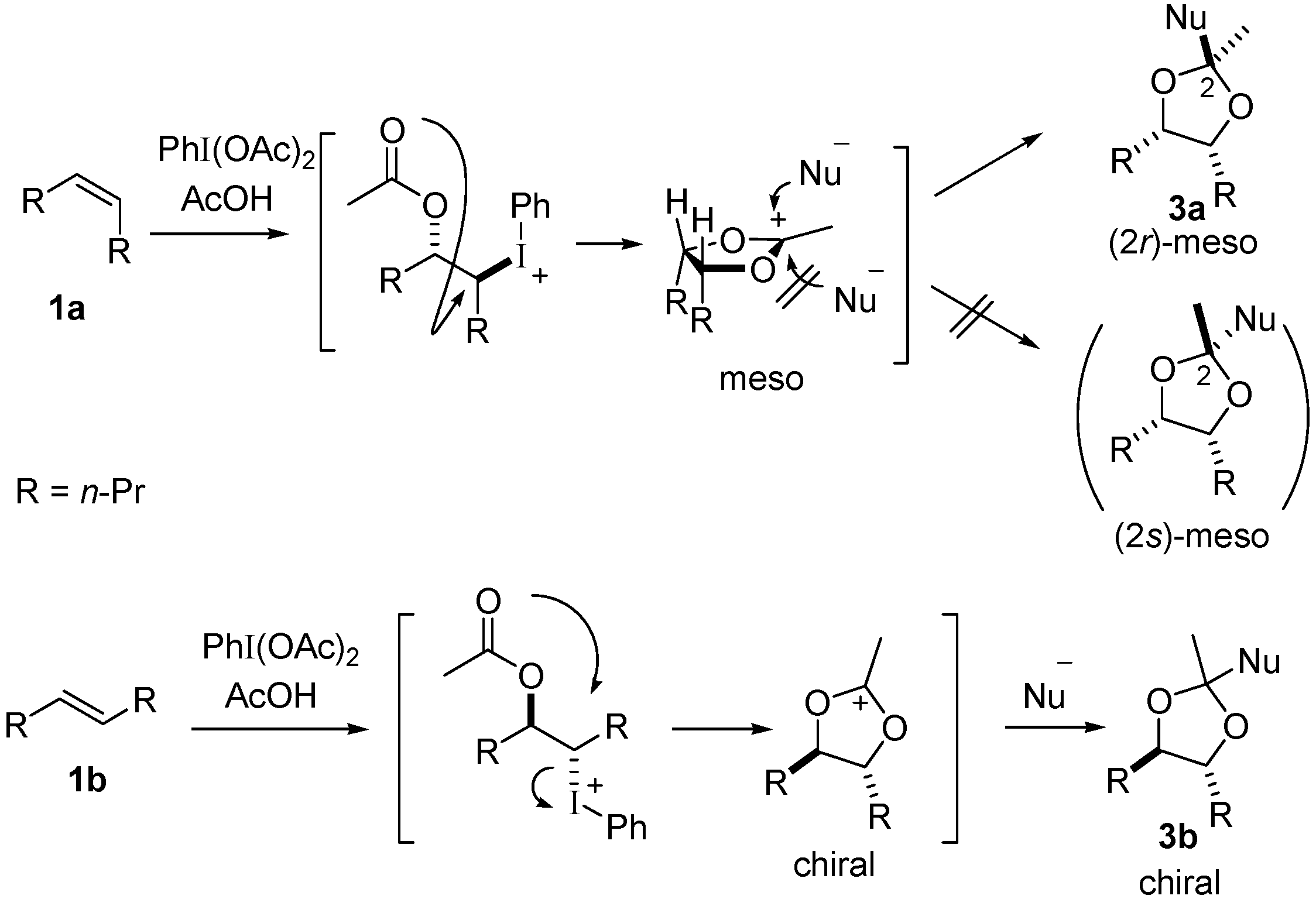
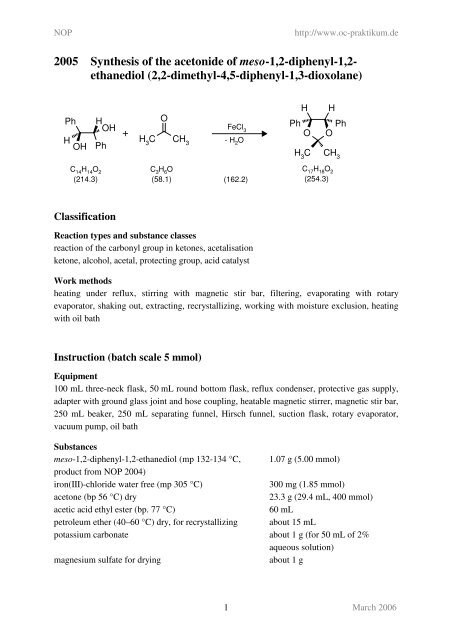
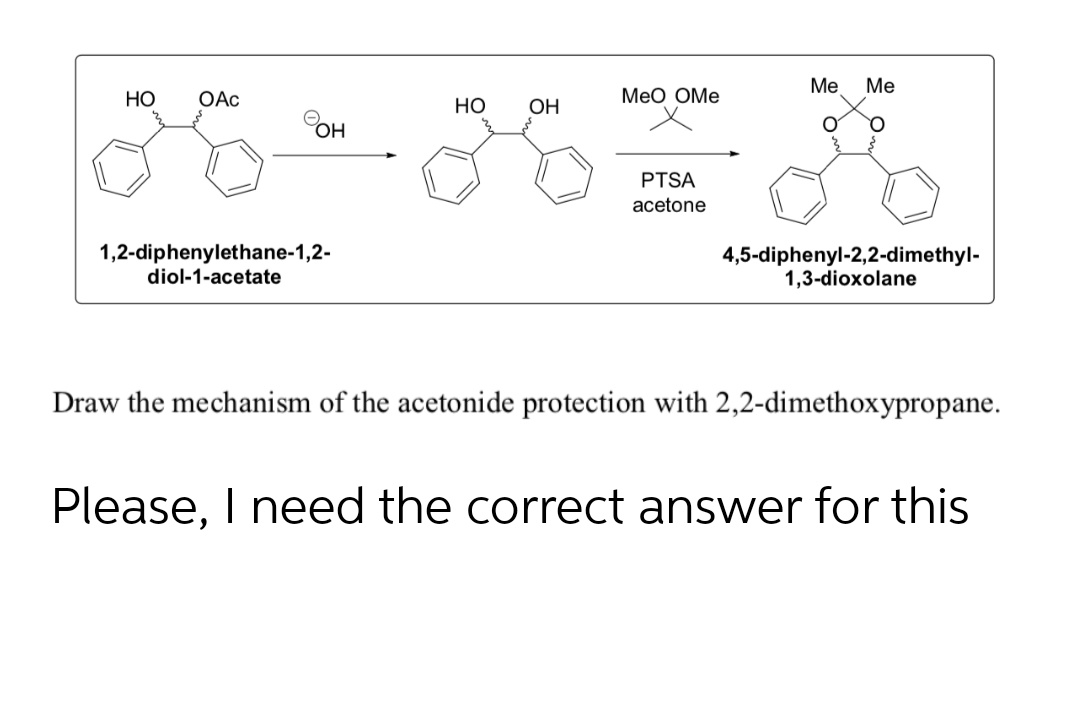
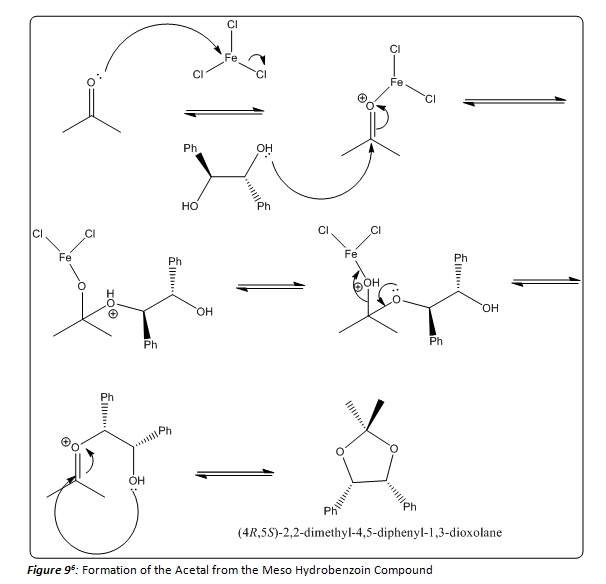
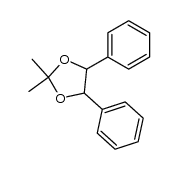
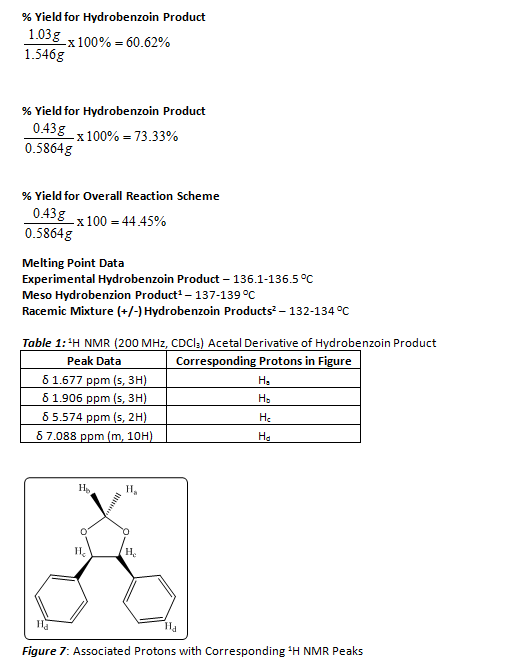
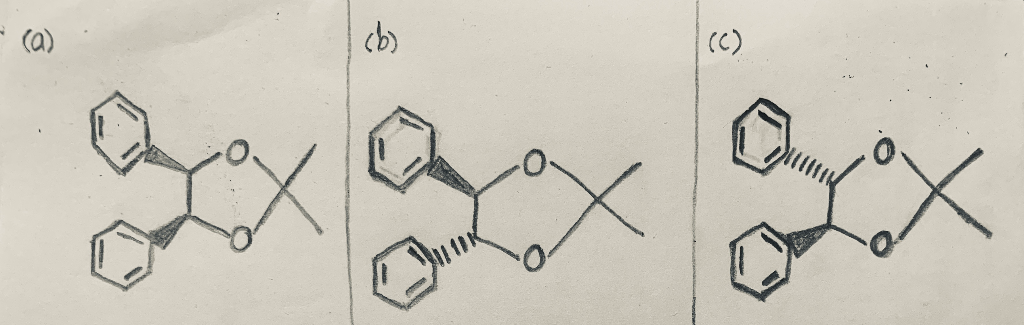
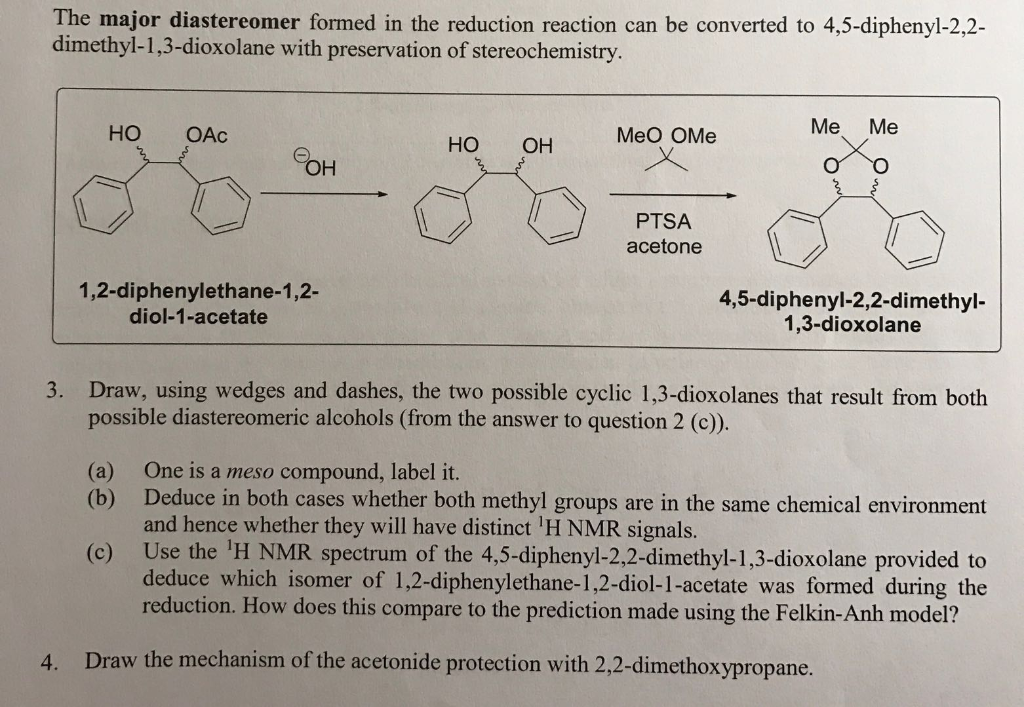
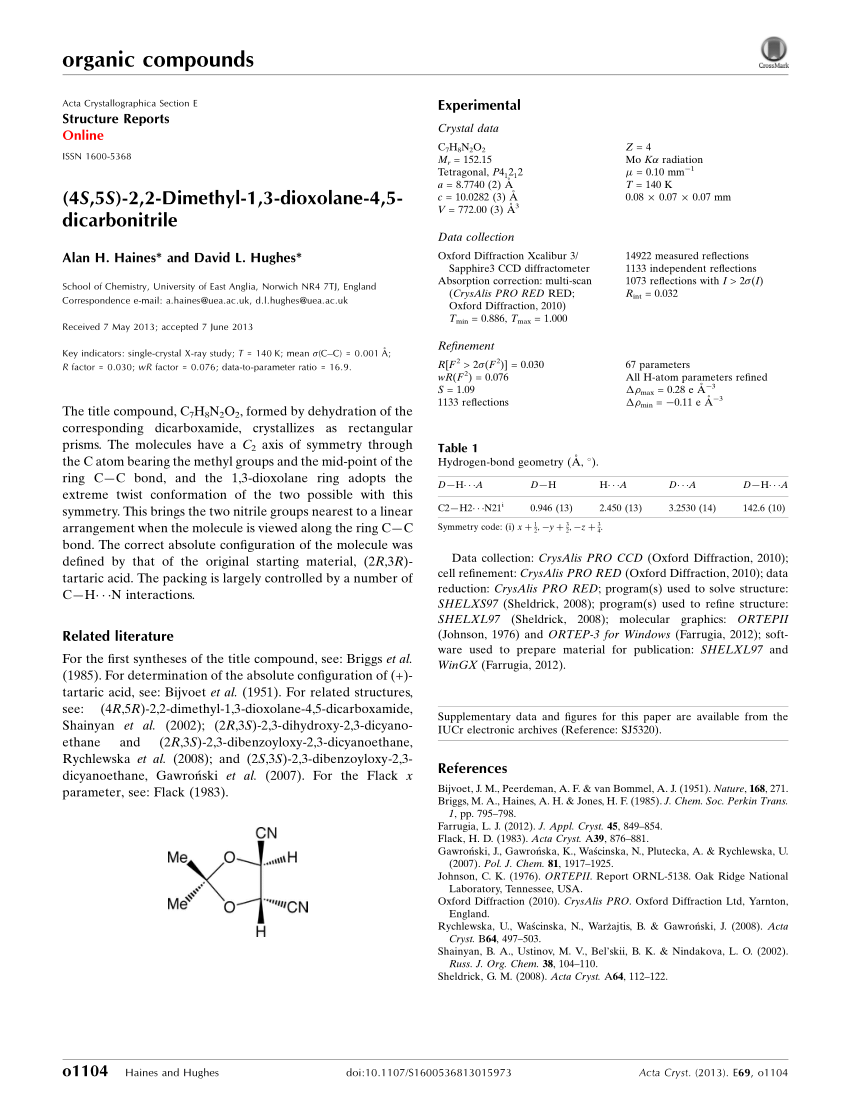
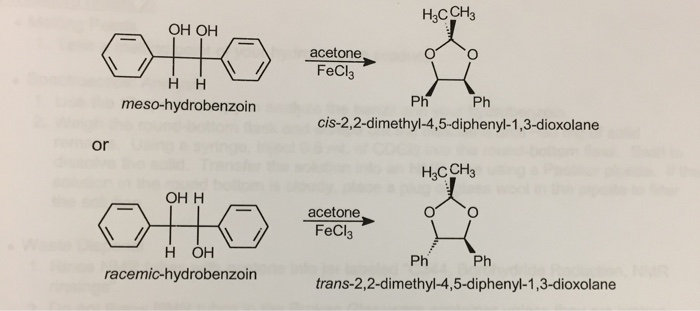
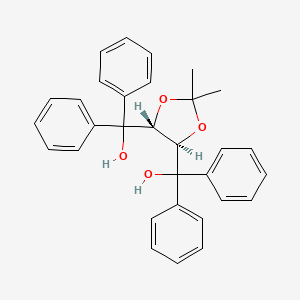
ORGANIC SPECTROSCOPY INTERNATIONAL: Synthesis of the acetonide of meso-1,2- diphenyl-1,2-ethanediol (2,2-dimethyl-4,5-diphenyl-1,3-dioxolane)
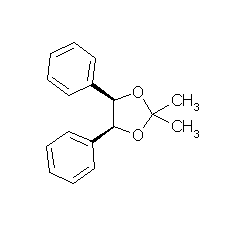
ORGANIC SPECTROSCOPY INTERNATIONAL: Synthesis of the acetonide of meso-1,2- diphenyl-1,2-ethanediol (2,2-dimethyl-4,5-diphenyl-1,3-dioxolane)

China Customized 1-Phenylvinylboronic Acid, Pinacol Ester 143825-84-7 Suppliers, Manufacturers, Factory - ALLYCHEM
ORGANIC SPECTROSCOPY INTERNATIONAL: Synthesis of the acetonide of meso-1,2- diphenyl-1,2-ethanediol (2,2-dimethyl-4,5-diphenyl-1,3-dioxolane)