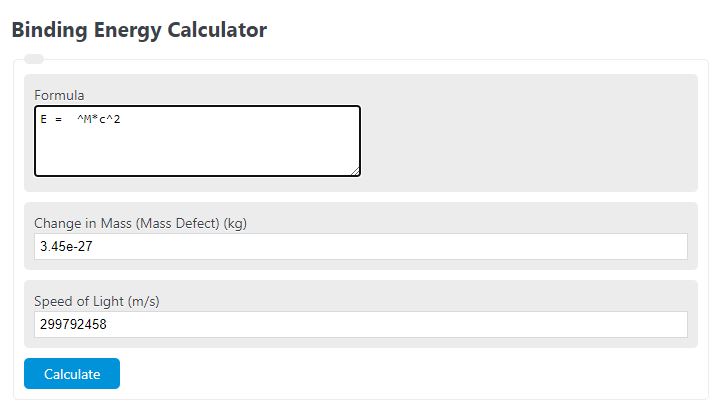
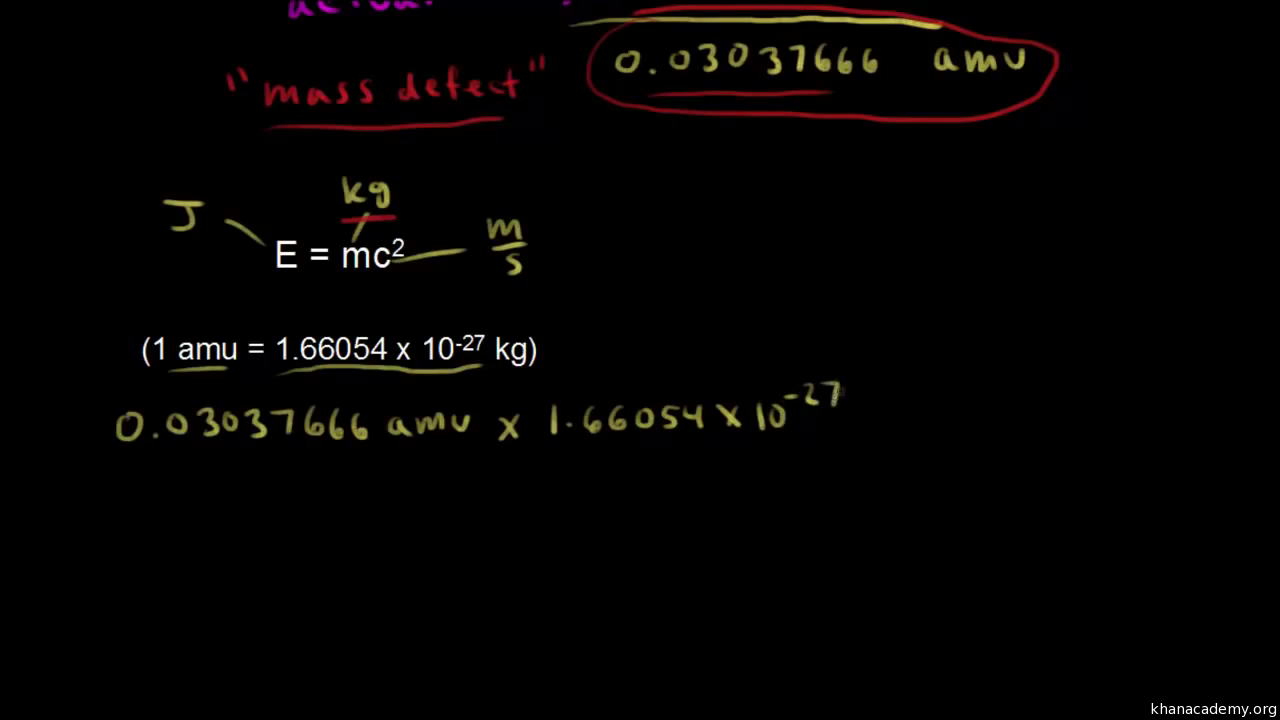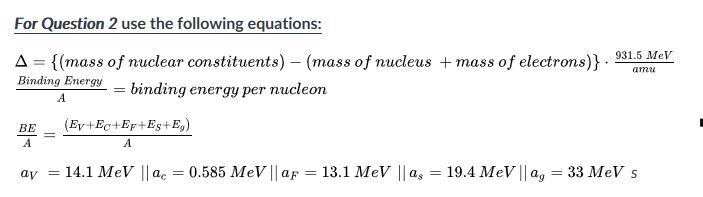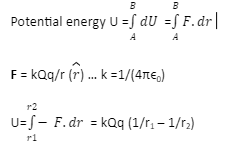Calculate the (i) mass defect, (ii) binding energy and (iii) the binding energy per nucleon for a 6C^12 nucleus. Nuclear mass of 6C^12 = 12.000000 a.m.u., mass of hydrogen nucleus = 1.007825

Mass Defect Formula & Examples | What is Nuclear Mass Defect? - Video & Lesson Transcript | Study.com
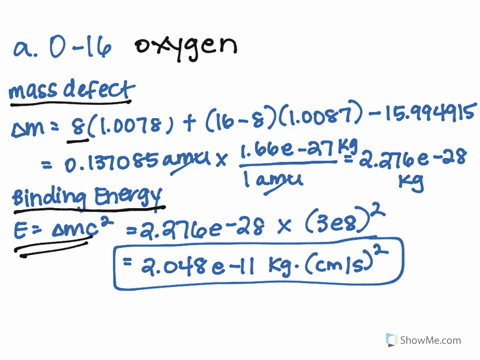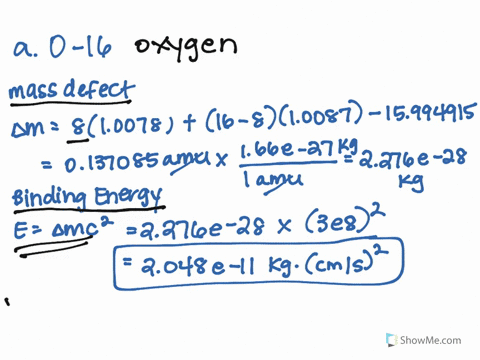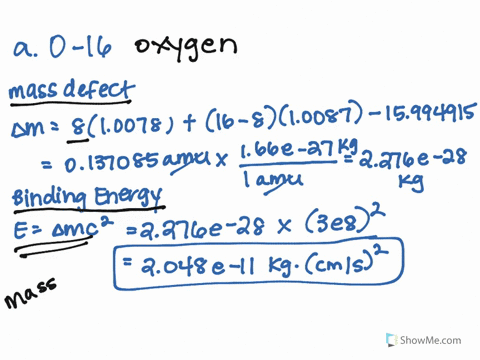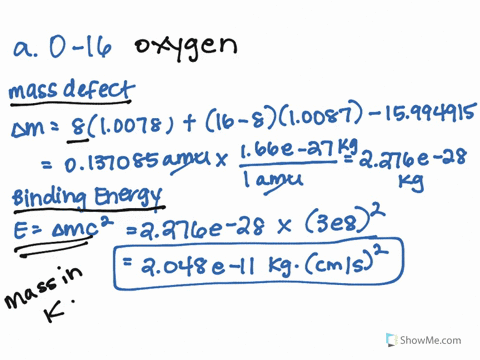
SOLVED: 1.) The mass of an 16 O atom is 15.994 91amu. Calculate the mass defect for the formation of an oxygen-16 nucleus in both grams and g/mol. 2.) Calculate the binding