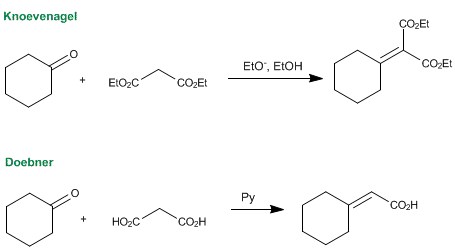
Contribution of Knoevenagel Condensation Products toward the Development of Anticancer Agents: An Updated Review - Tokala - 2022 - ChemMedChem - Wiley Online Library

A Stereoselective and Practical Synthesis of (E)‐α,β‐Unsaturated Ketones from Aldehydes - Balducci - 2011 - European Journal of Organic Chemistry - Wiley Online Library

Proline-Mediated Knoevenagel–Doebner Condensation in Ethanol: A Sustainable Access to p-Hydroxycinnamic Acids | ACS Sustainable Chemistry & Engineering

Proline-Mediated Knoevenagel–Doebner Condensation in Ethanol: A Sustainable Access to p-Hydroxycinnamic Acids | ACS Sustainable Chemistry & Engineering

Synthesis of Acrylamides via the Doebner–Knoevenagel Condensation | The Journal of Organic Chemistry

Microwave-Assisted Knoevenagel-Doebner Reaction: An Efficient Method for Naturally Occurring Phenolic Acids Synthesis. - Abstract - Europe PMC

Quinoline synthesis methods: Skraup reaction (A); Doebner reaction (B);... | Download Scientific Diagram

Combes quinoline synthesis Doebner–Miller reaction Doebner reaction Reaction mechanism Organic synthesis, creative chin, png | PNGEgg
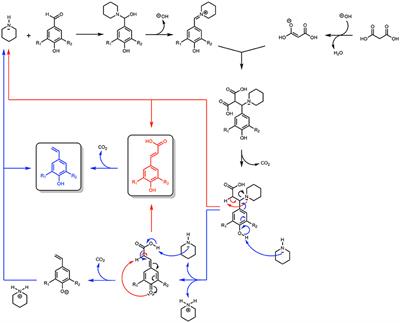
Frontiers | Microwave-Assisted Knoevenagel-Doebner Reaction: An Efficient Method for Naturally Occurring Phenolic Acids Synthesis