PPT - Large sample dose content uniformity test: parametric and nonparametric (counting) PowerPoint Presentation - ID:9186110

PPT – Large%20sample%20dose%20content%20uniformity%20test:%20parametric%20and%20nonparametric%20(counting) PowerPoint presentation | free to download - id: 7547b1-YmZjY

Uniformity of dosage units—comparative study of methods and specifications between Eur. Pharm. 3rd and USP 23 - ScienceDirect
ICH guideline Q4B annex 6 to note for evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on unifor

Factor analysis in optimization of formulation of high content uniformity tablets containing low dose active substance - pharma excipients
Updates of Ph. Eur. dosage form monographs and general chapters – Users invited to comment in Pharmeuropa 33.1 - European Directorate for the Quality of Medicines & HealthCare
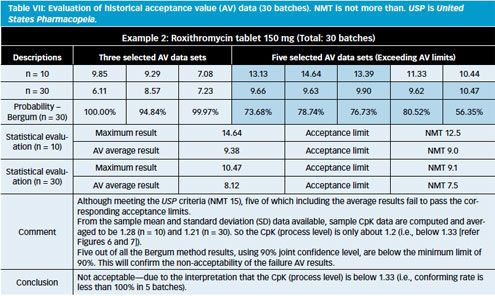
![PDF] Evaluation of the Discrepancy between the European Pharmacopoeia Test and an Adopted United States Pharmacopoeia Test Regarding the Weight Uniformity of Scored Tablet Halves: Is Harmonization Required? | Semantic Scholar PDF] Evaluation of the Discrepancy between the European Pharmacopoeia Test and an Adopted United States Pharmacopoeia Test Regarding the Weight Uniformity of Scored Tablet Halves: Is Harmonization Required? | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0219ae49f3ffe1a993893eabe666b5a77ba0a4e2/3-TableI-1.png)
PDF] Evaluation of the Discrepancy between the European Pharmacopoeia Test and an Adopted United States Pharmacopoeia Test Regarding the Weight Uniformity of Scored Tablet Halves: Is Harmonization Required? | Semantic Scholar
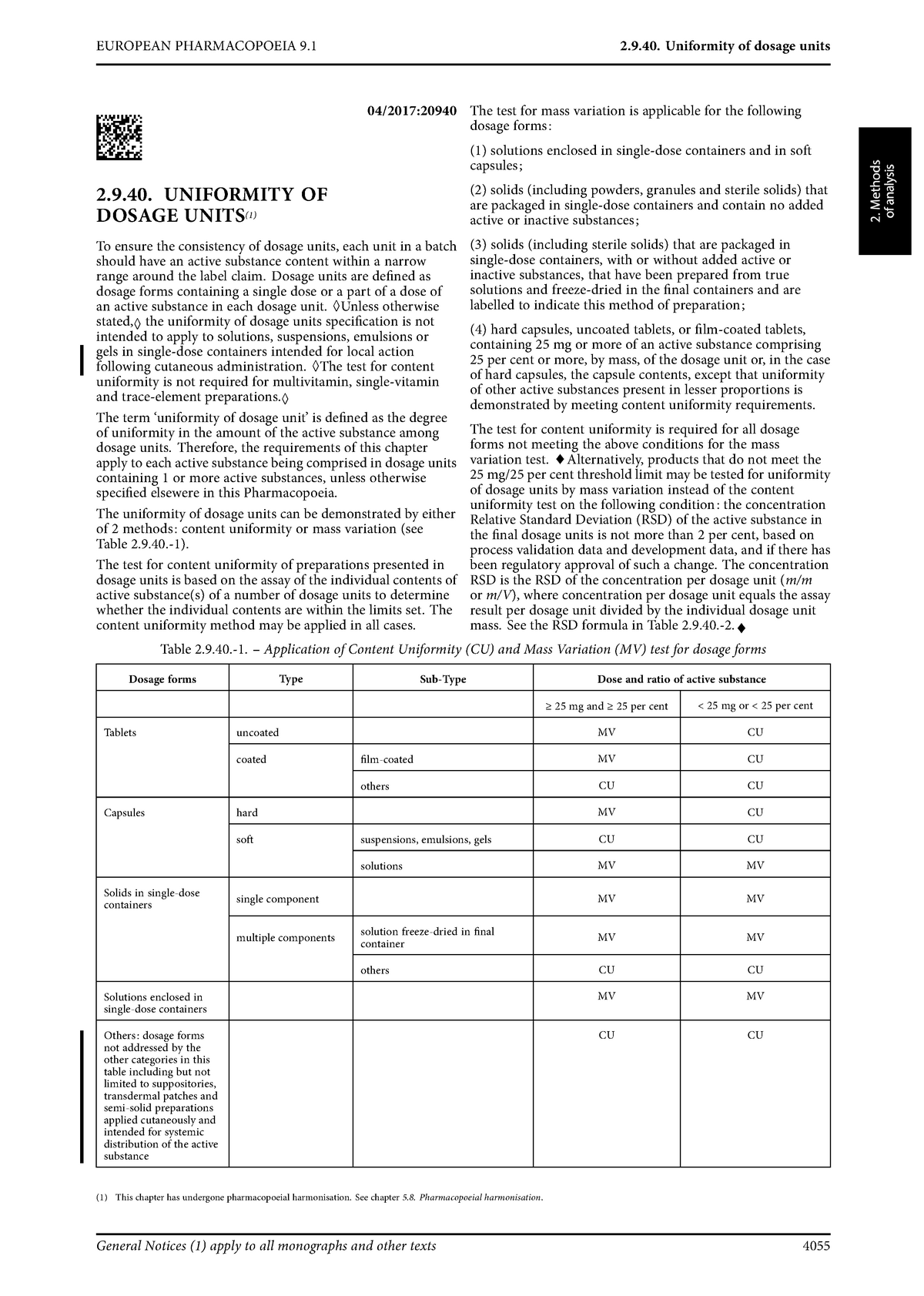
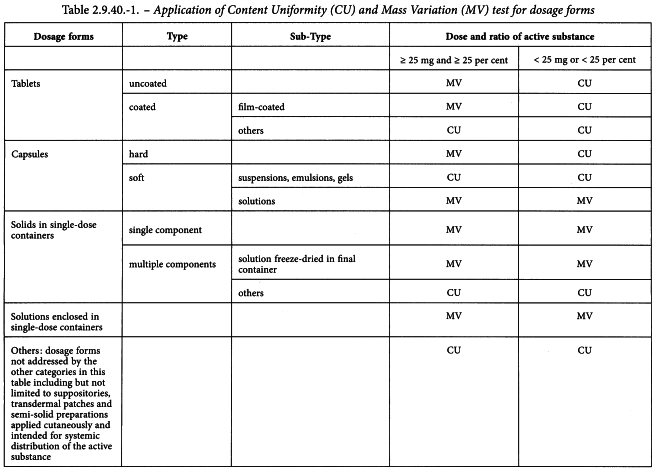
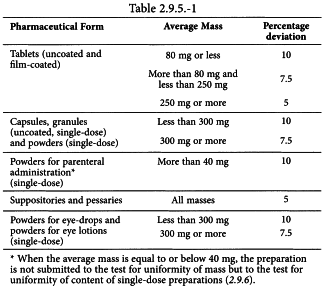
Uniformity of dosage units - EUROPEAN PHARMACOPOEIA 9 2.9. Uniformity of dosage units 04/2017: 2.9. - Studocu